The cells of all residing organisms are powered by the identical chemical gas: adenosine triphosphate (ATP). Now, researchers have discovered a solution to generate ATP immediately from electrical energy, which may turbocharge biotechnology processes that develop every little thing from meals to gas to prescribed drugs.
Interfacing fashionable electronics-based expertise with biology is notoriously troublesome. One main stumbling block is that the best way they’re powered could be very totally different. Whereas most of our devices run on electrons, nature depends on the power launched when the chemical bonds of ATP are damaged. Discovering methods to transform between these two very totally different currencies of power may very well be helpful for a number of biotechnologies.
Genetically engineered microbes are already getting used to supply varied high-value chemical compounds and therapeutically helpful proteins, and there are hopes they may quickly assist generate greener jet gas, break down plastic waste, and even develop new meals in large bioreactors. However on the minute, these processes are powered by an inefficient means of rising biomass, changing it to sugar, and feeding it to the microbes.
Now, researchers on the Max Planck Institute for Terrestrial Microbiology in Germany have devised a way more direct solution to energy organic processes. They’ve created a man-made metabolic pathway that may immediately convert electrical energy into ATP utilizing a cocktail of enzymes. And crucially, the method works in vitro and doesn’t depend on the native equipment of cells.
“Feeding electrical energy immediately into chemical and biochemical reactions is an actual breakthrough,” Tobias Erb, who led the analysis, stated in a press launch. “It will allow synthesis of energy-rich helpful sources akin to starch, biofuels, or proteins from easy mobile constructing blocks—sooner or later even from carbon dioxide. It might even be doable to make use of organic molecules to retailer electrical power.”
In nature, ATP and its sister molecule adenosine di-phosphate (ADP) may be regarded as virtually like batteries. ATP is sort of a charged battery, storing power in its chemical bonds. If a cell must spend that power, it breaks off one of the molecule’s three phosphate teams and the power certain up in that chemical bond can then energy some mobile course of.
This course of converts the ATP molecule into ADP, which may be regarded as an empty battery. To recharge it, the cell wants to make use of power from meals or photosynthesis so as to add a phosphate group again onto the ADP molecule, turning it again into ATP.
However this recharging course of depends on a posh sequence of reactions involving varied protein complexes embedded within the cell membrane. Re-engineering this technique to work exterior of a cell is difficult as a result of it requires the assorted proteins to be rigorously oriented in a man-made membrane, which makes it each finicky and fragile.
The brand new strategy, outlined in a paper in Joule, is way less complicated. Dubbed the “AAA cycle,” it includes simply 4 enzymes interacting in an answer. The important thing ingredient that made all of it doable was the invention of an enzyme known as aldehyde ferredoxin oxidoreductase (AOR) in a recently-discovered bacterium known as Aromaticum aromatoleum, which is ready to break down petroleum.
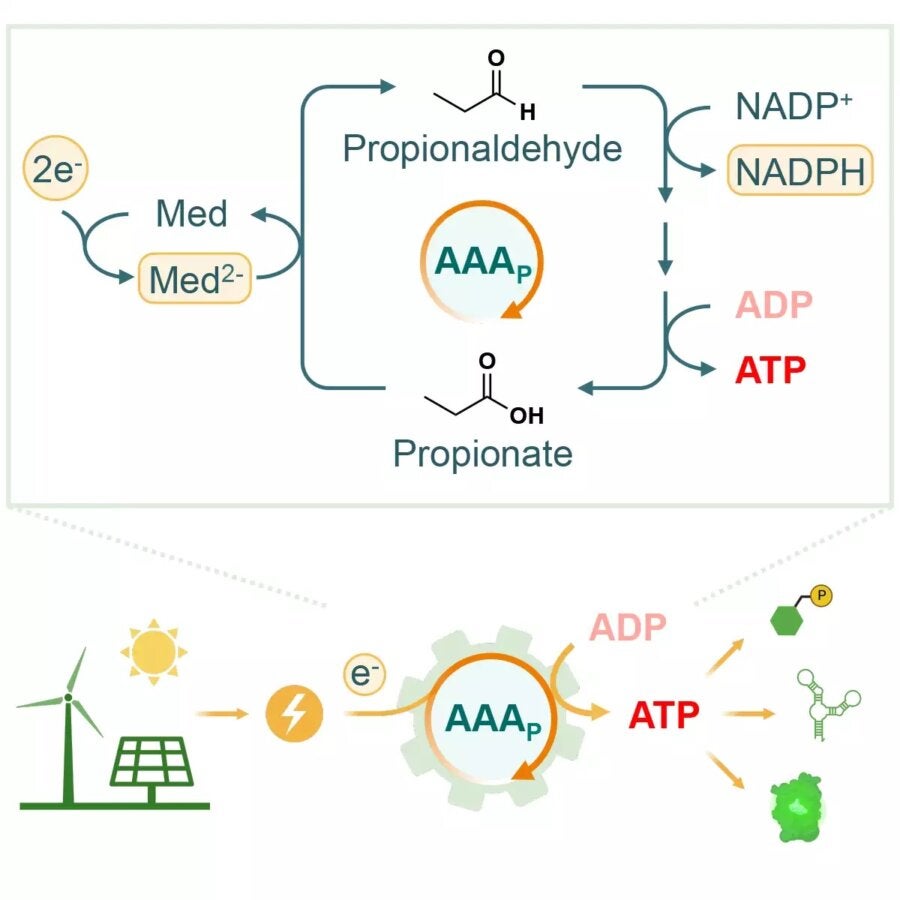
This enzyme is ready to take the electrons from an electrode and bind up their power in an aldehyde bond that’s added to a precursor chemical known as propionate. That is then cascaded by three extra enzymes that act on the chemical and in the end use the power saved in it to transform ADP to ATP. On the finish, a propionate molecule pops out that may then be fed again into the cycle.
“The straightforward AAA cycle is a intelligent and stylish strategy…that’s a lot less complicated than how biology naturally makes ATP,” Drew Endy, an artificial biologist at Stanford College, advised Science. He added that it may very well be a key enabler to make “electrobiosynthesis” doable, the thought of utilizing electrical energy to immediately energy the synthesis of helpful chemical compounds by cells.
The researchers say the method nonetheless wants work, because the enzymes are unstable and solely capable of convert a small quantity of power. But when the thought may be refined and scaled up, it may make it doable to run every kind of highly effective biotechnology processes on renewable power, not solely making them greener however considerably increaseing the quantity of power they will faucet into.
Picture Credit score: MPI for Terrestrial Microbiology/ Virginia Geisel

